Code of Federal Regulations, Title 21 Food and Drugs 1300-End, Revised as of April 1, 2021 - 9781636718415
.jpg)
FDA CFR Title 21 Food and Drugs Regulations - TELUGU GMP - Provides GMP Pharmaceutical Guidelines in Telugu.

The Complete Code of Federal Regulations, Title 21, Food And Drugs, FDA Regulations, 2016 - Kindle edition by United States Government. Professional & Technical Kindle eBooks @ Amazon.com.

Amazon | CODE OF FEDERAL REGULATIONS TITLE 21 Food And Drugs BUDGET EDITION 2018 PARTS 1-99: CFR TITLE 21 | FEDERAL REGISTER, OFFICE OF THE | Law

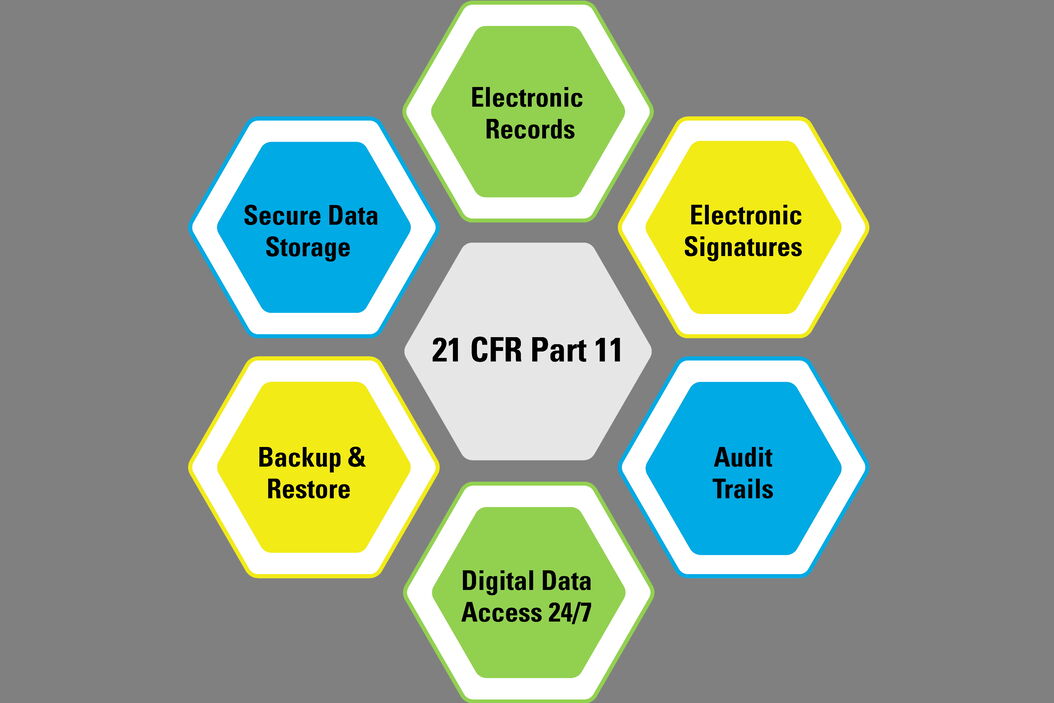
FDA CFR Part 11 Compliance | Security Assessment | Compliance Services | Certification & Attestation | DIY Platform

Book M2: 2022 Mini Pocket-Sized (3" x 5") Code of Federal Regulations – Clinical Research Resources, LLC